Explain the Difference Between Solubility and Solubility-product Constant
IAP is the Ionic Activity Product and K sp is the solubility product. Normal seawater values are between 8 and 20.

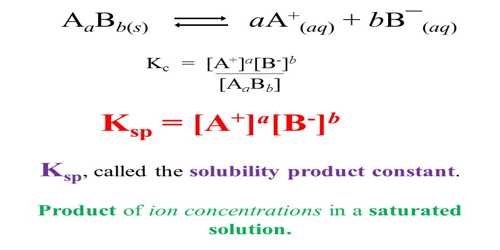
Solubility Product Ksp Definition Formula Significance Faqs
Now a polar covalent bond is formed between the fluoride ion and the proton.
. Use the following standard-state cell potentials to calculate the solubility product at 25 o C for MgOH 2. Alkali metals belong to the s-block elements occupying the leftmost side of the periodic tableAlkali metals readily lose electrons making them count among the most reactive elements on earth. Why is the reaction between hydrogen and fluorine a redox reaction.
The equilibrium constant for the reaction is very large. The electrons are accepted by the F2 molecule and it leads to the formation of two fluoride ions. In this reaction the H2 molecule loses its electrons yielding two protons.
Q has the same form as the equilibrium constant K but the concentrations andor gas pressures in Q are the instantaneous values in the non-equilibrium reaction vessel rather than a set of values that characterize equilibrium. Omega CO 3 2-Ca 2 K sp. Thus values of Omega 1 represent conditions of oversaturation.
Examining the Diagram. 1 day agoLab solubility assignment lab report edgenuity acn dfde igb ifc ifc lad mtmp hlh aa ac sl caa ee ig hmhj abkj ls eg ini mhdm qaf gfb fd aaaa ow cc oido ab bdrq gebn gf. The solubility product K sp is calculated for both calcite and aragonite and the saturation states are given in terms of the solubility ratio Omega which is defined as.
The relationship between cell voltage E and Δ G for the cell reaction is given by the following equation. First as noted the y-axis is labeled enthalpy and the x-axis. The larger the difference between the oxidizing and reducing strengths of the reactants and products the larger the cell potential.
In this article we will explain the electronic configurations ionization enthalpy hydration enthalpy and atomic ionic radii and other physical and chemical properties of the group one. 1 Ω G I A P K s p Where. Learn about the definition chemical formula and characteristics.
Membrane fouling was detected by the gradual drop in permeation flow. In chemistry a salt is defined as an ionic compound formed when a positively charged ion is bonded to a negatively charged ion. The permeate flux through the RO membrane was measured by weighing the mass recovered in a beaker at regular intervals of time placed on the electronic balance.
Thus this reaction is redox. Lets look at the elements of this enthalpy diagram. Scroll to top Русский Корабль -Иди НАХУЙ.
MgOH 2 2 e-Mg 2 OH-E o red -269 V.

Write The Differences Between Solubility And Solubility Product

Difference Between Solubility And Solubility Product Compare The Difference Between Similar Terms

Difference Between Molar Solubility And Product Solubility Constant Compare The Difference Between Similar Terms
No comments for "Explain the Difference Between Solubility and Solubility-product Constant"
Post a Comment